Specific Heat Ratio of Real Gas
Equation of state is used to derive variety of thermodynamic properties. This article illustrate calculation of specific heat ratio from Peng Robinson Equation of state.
Example
Calculate specific heat ratio ( γ = Cp/Cv ) for methane gas at 11 Bar & 300 °K. Critical constants for Methane are as following
- Critical temperature, Tc : 190.6°K
- Critical Pressure, Pc : 46.002 bar
- Accentric Factor, ω : 0.008
Ideal gas specific heat constants CpIG = A + B.T + C.T² + D.T³ are as following
- A = 4.5980
- B = 0.0125
- C = 2.86 x 10-6
- D = -2.7 x 10-9
where Cp is in cal/mol-K
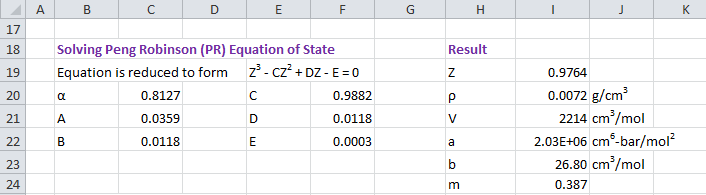
Peng Robinson equation of state is defined as
P = RT / (V - b) - a / [V(V + b) + b(V - b)]where
ac = 0.45723553 R²Tc²/Pcb = 0.077796074 RTc/Pcm = 0.37464 + 1.54226ω - 0.26992ω²a = ac[1 + m(1 - (T/Tc)0.5)]²
Above equation is translated into polynomial form and solved for values of Z using Newton-Raphson method.
Z³ - (1 - B)Z² + Z (A - 2B - 3B²) - (AB - B² - B³) = 0Z = PV/RTA = aP/ (RT)²B = bP/ RT
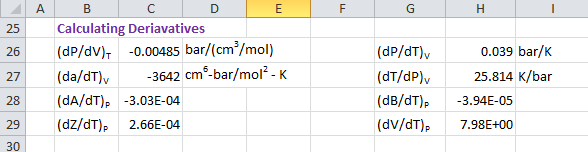
Following partial derivatives are required for calculating thermodynamic properties. First derivative is obtained by differentiation of P with respect to V at constant T.
(δP/ δV)T = -RT/(v - b)² + 2a(v + b)/[v(v + b) + b(v - b)]²(δP/ δV)T = -0.00485 bar/(cm3/mol)
Second derivative is obtained by differentiation of P with respect to T at constant V.
(δP/ δT)V = R/(v - b) - a'/[v(v + b) + b(v - b)](δa/ δT)V = -mac/[(TTc)0.5(1 + m( 1 - (T/Tc)0.5))](δP/ δT)V = 0.039 bar/K(δT/ δP)V = 25.814 K/bar
Third derivative is obtained by differentiation of V with respect to T at constant P.
(δV/ δT)P = (R/P)[ T(δZ/δT)P + Z](δZ/ δT)P = Num / DenomNum = (δA/δT)P (B-Z) + (δB/δT)P(6BZ+2Z-3B²-2B+A-Z²)Denom = 3Z² + 2(B-1)Z + (A-2B-3B²)
where,
(δA/δT)P = (P/(RT)²)(a' - 2a/T)(δB/δT)P = -bP/(RT²)
Calculation of Heat Capacities
Ideal gas heat capacity CpIG is calculated at 300 °K from polynomial equation provided above. Specific heat at constant volume for ideal gas, CvIG is calculated using following relation.
CvIG = CpIG - RResidual heat capacity at constant volume Cv R is calculated from internal energy U R as following.
CvR = (δUR/δT)VUR = [(Ta'-a)/b(8)0.5] ln[(Z+B(1+20.5))/(Z+B(1-20.5))]CvR = [Ta"/b(8)0.5] ln[(Z+B(1+20.5))/(Z+B(1-20.5))]
where,
a" = ac m (1 + m)(Tc/T)0.5/ (2TTc)Specific heat capacity at constant pressure and volume is calculated using following equation.
CpR = CvR + T(δP/δT)V(δV/δT)P - RCp = CpIG + CpRCv = CvIG + CvR
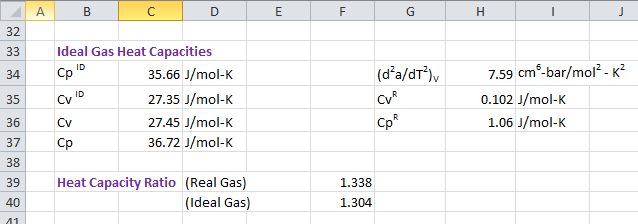
Specific heat ratio is obtained as :
γ = Cp / Cvγ = 1.338
Resources
- Spreadsheet for Specific heat ratio calculaton from Peng Robinson EOS