Solving Cubic Equation of State
Equation of State are used to predict pure component and mixture properties such as compressibility, fugacity and mixture equilibrium.
Soave - Redlich - Kwong (SRK) EOS
Equation is defined as
P = RT / (V - b) - a α / V(V + b)where
a = 0.42748 R²Tc²/Pcb = 0.08664 RTc/Pcα = [1 + (0.48 + 1.574ω - 0.176ω² )(1 - Tr0.5)]²
Above equation is translated into polynomial form.
Z³ - Z² + Z (A - B - B²) - AB = 0Z = PV/RTA = 0.42748 α Pr/ Tr²B = 0.08664 Pr/ Tr
where, Pr is Reduced Pressure (= P / Pc), Tr is Reduced Temperature (= T / Tc).
Newton-Raphson Method
Newton Raphson is an iterative procedure for finding roots of a function f(Z). Function f(Z) and its derivative f '(Z) is calculated. An initial guess is made for the root Z, successive vales for Z' are estimated using below relation till there is negligible difference between successive Z values.
f(Z) = Z³ - Z² + Z (A - B - B²) - ABf'(Z) = 3Z² - 2Z + (A - B - B²)Z' = Z - f(Z)/f'(Z)
Example
Calculate compressibility factor for Methane based on SRK EOS at 30 bar, 285 °K. Critical parameters are Tc : 190.6 °K, Pc : 46 Bar, ω : 0.008.
Based on above equations f(Z) & f'(Z) is calculated as following.
f(Z) = Z³ - Z² + 0.0596 Z - 0.0037f'(Z) = 3Z² - 2Z + 0.0596
It is solved iteratively using Newton-Raphson method.
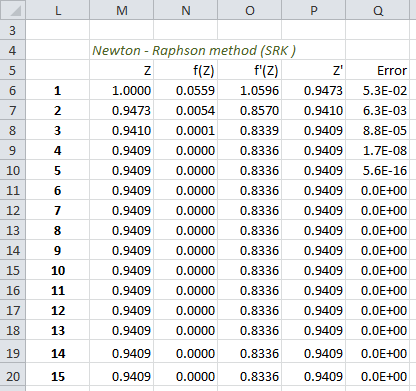
There is negligible error in successive values of Z after 6th iteration.
Z = 0.9409Peng - Robinson (PR) EOS
Equation is defined as
P = RT / (V - b) - a α / [V(V + b) + b(V - b)]where
a = 0.45724 R²Tc²/Pcb = 0.07780 RTc/Pcα = [1 + (0.37464 + 1.54226ω - 0.26992ω² )(1 - Tr0.5)]²
Above equation is translated into polynomial form.
Z³ - (1 - B)Z² + Z (A - 2B - 3B²) - (AB - B² - B³) = 0Z = PV/RTA = 0.45724 α Pr/ Tr²B = 0.07780 Pr/ Tr
where, Pr is Reduced Pressure (= P / Pc), Tr is Reduced Temperature (= T / Tc). Above relation is then solved for values of Z using Newton-Raphson method.
Resources
- Spreadsheet for Solving cubic Equation of State