Estimating Binary Interaction Parameter for Peng Robinson EOS
This article shows how to estimate binary interaction parameter (BIP) used in Peng Robinson (PR) Equation of State (EOS) from experimental data by regression in excel spreadsheet.
Example
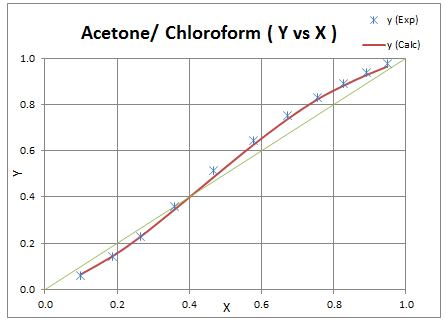
Determine binary interaction parameter used in Peng Robinson EOS for a mixture of acetone and chloroform from Tx-y experimental data available at 101.33 kPa.
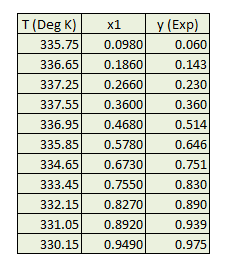
Obtain pure component properties like critical temperature (Tc), critical pressure (Pc) and accentric factor ω from literature. Experimental K1 is determined from x, y data.
K1Experimental = y1 / x1Peng Robinson EOS parameters are calculated.
κi = 0.37464 + 1.54226ω - 0.26992ω²αi = [ 1 + κi (1 - (T/Tc)0.5)]²ai = 0.45724 (RTc)²α / Pcbi = 0.07780 RTc / Pc
Mixture parameters are calculated next
a12= [(a1.a2)0.5(1 - k12)] = a21a = a1.x1² + 2.a12.x1.x2 + a2.x2²b = b1.x1 + b2.x2A = aP/(RT)²B = bP/RT
where k12 is Binary Interaction Parameter (BIP) to be estimated. For first run, use a guess value to start calculation.
Following cubic equation is solved to get ZL.
Z³ + (B-1)Z² + (A-3B² -2B)Z + (B³+B²-AB) = 0Above equation can be written as
Z³ + C2.Z² + C1.Z + C0 = 0Solving Cubic Equation
Cubic equation is solved using following procedure. Calculate Q1, P1 & D.
Q1 = C2.C1/6 - C0/2 - C2³/27P1 = C2²/9 - C1/3D = Q1² - P1³
If D >= 0, then equation has only one real root provided by
Z1 = (Q1 + D0.5)1/3 + (Q1 - D0.5)1/3 - C2/3If D < 0, then equation has 3 real roots, following parameters are calculated
t1 = Q1² / P1³t2 = (1 - t1)0.5 / t10.5. Q1/abs(Q1)θ = atan(t2)
Roots are calculated as following -
Z0 = 2.P10.5.cos(θ/3) - C2/3Z1 = 2.P10.5.cos((θ + 2*Π)/3) - C2/3Z2 = 2.P10.5.cos((θ + 4*Π)/3) - C2/3
Roots thus calculated are arranged in descending order, highest root gives ZV and lowest root gives ZL.
Fugacity
Based on ZL, liquid fugacity φiL is calculated for each data set.
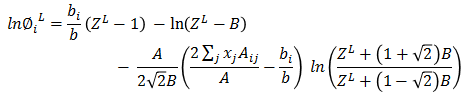
In next step, Vapor phase fugacity is calculated. Mixture properties are estimated as following
a = a1.y1² + 2.a12.y1.y2 + a2.y2²b = b1.y1 + b2.y2A = aP/(RT)²B = bP/RT
Cubic equation is solved as per method shown above to get ZV.
Z³ + (B-1)Z² + (A-3B² -2B)Z + (B³+B²-AB) = 0Based on ZV, vapor fugacity φiV is calculated for each data set.
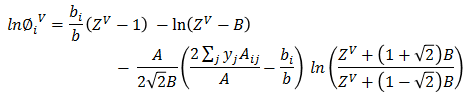
K1 is calculated as:
K1Calculated = φ1L/φ1VDifference of K1Experimental and K1Calculated is obtained for all data points. An objective function is defined as summation of all these differences. Click on Solver in Data Ribbon (Excel 2010) to open dialog box for Solver parameters and input data as shown below.
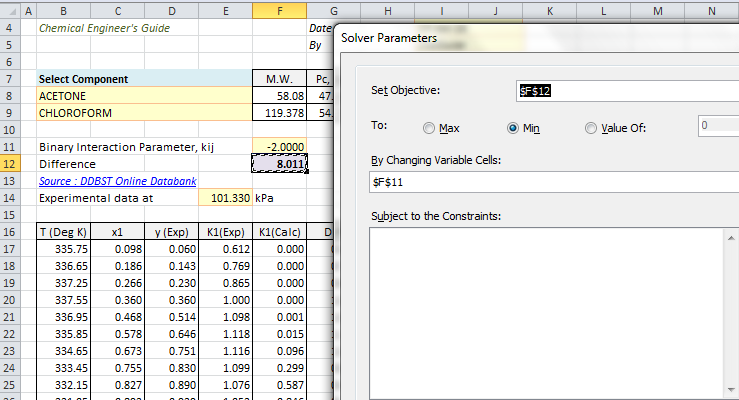
Minimize the objective function by changing values of kij. Uncheck Make Unconstrained Variables Non-Negative, as k12 can take negative value. Click solve to start regression and new value k12 is calculated.
k12 = -0.0605Above value is then used to calculate y1 values and results are plotted to check the deviation.
Example
Determine temperature dependent binary interaction parameter used in Peng Robinson EOS ( k12 = k1 + k2.T + k3/T ) for a mixture of acetone and n-Hexane from P x-y experimental data available at 318.15 °K.
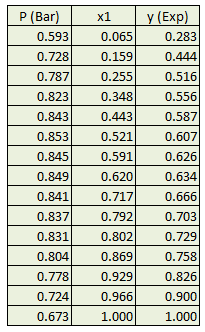
Follow above steps to calculate pure component and mixture parameters. Change formula for kij to make it temperature dependent. As an initial guess put values for 3 parameters (k1,k2,k3) which gives a numeric difference value. In solver select these 3 variables to be varied for minimizing the difference value and then solve it. After doing regression binary interaction parameters are obtained and result is plotted as following.
k1 = -0.0218k2 = 3.142x10-4k3 = 10.155
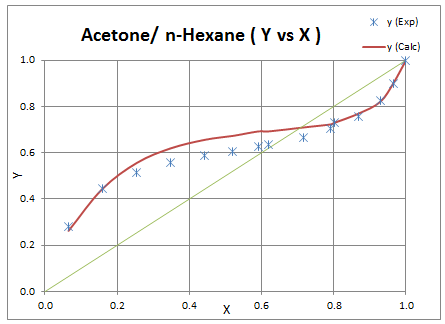
Note : In case of non-convergence, try different initial guess values for kij and then let solver obtain optimized values.
Resources
- Spreadsheet for Regressing BIP for Peng Robinson EOS