Bubble P Flash using PR EOS
Bubble P flash calculation determine bubble point pressure (P) and vapor mol fraction (Yi) for a mixture at given temperature (T) and liquid mol fraction (Xi). These calculations can be performed in excel spreadsheet using Peng Robinson Equation of State (PR EOS).
Estimate pressure P and vapor mol fraction (Yi). P can be estimated as following -
P = Σ Pisat XiPisat = exp[ ln(Pc) + ln(10)(7/3)(1 + ω )(1-Tc/T)]
where Pc, Tc and ω are critical constants for a component i. Vapor mol fraction is estimated as following
Yi = Ki XiKi = exp[ ln(Pc/P) + ln(10)(7/3)(1 + ω )(1-Tc/T)]
First iteration starts with estimated P and Yi. Parameters for Peng Robinson EOS are calculated for each component i.
κi = 0.37464 + 1.54226ω - 0.26992ω²αi = [ 1 + κi (1 - (T/Tc)0.5)]²ai = 0.45724 (RTc)²α / Pcbi = 0.07780 RTc / Pc
Mixture parameters are calculated next
aij = [(ai.aj)0.5(1 - kij)] = ajia = ΣiΣj aij.Xi.Xjb = Σi bi.XiA = aP/(RT)²B = bP/RT
where, kij's are Binary Interaction Parameter available from literature.
Following cubic equation is solved to get ZL.
Z³ + (B-1)Z² + (A-3B² -2B)Z + (B³+B²-AB) = 0Above equation can be written as
Z³ + C2.Z² + C1.Z + C0 = 0Solving Cubic Equation
Cubic equation is solved using following procedure. Calculate Q1, P1 & D.
Q1 = C2.C1/6 - C0/2 - C2³/27P1 = C2²/9 - C1/3D = Q1² - P1³
If D >= 0, then equation has only one real root provided by
Z1 = (Q1 + D0.5)1/3 + (Q1 - D0.5)1/3 - C2/3If D < 0, then equation has 3 real roots, following parameters are calculated
t1 = Q1² / P1³t2 = (1 - t1)0.5 / t10.5. Q1/abs(Q1)θ = atan(t2)
Roots are calculated as following -
Z0 = 2.P10.5.cos(θ/3) - C2/3Z1 = 2.P10.5.cos((θ + 2*Π)/3) - C2/3Z2 = 2.P10.5.cos((θ + 4*Π)/3) - C2/3
Roots thus calculated are arranged in descending order, highest root gives ZV and lowest root gives ZL.
Fugacity
Based on ZL, liquid fugacity φiL is calculated for each component.
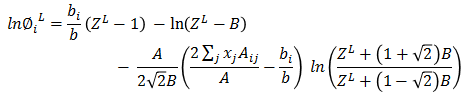
As a next step, Vapor phase fugacity is calculated. Mixture properties are estimated as following -
a = ΣiΣj aij.Yi.Yjb = Σi bi.YiA = aP/(RT)²B = bP/RT
Cubic equation is solved as per method shown above to get ZV.
Z³ + (B-1)Z² + (A-3B² -2B)Z + (B³+B²-AB) = 0Based on ZV, vapor fugacity φiV is calculated for each component.
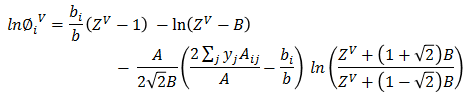
Vapor phase mol fraction is calculated as
Yi = Xi.φiL/φiVNew values of Yi thus calculated are again used to estimate φiV and thereafter Yi. This iteration is repeated till there is no further change in Yi values. Typically, in 25 iterations change in Yi values become negligible.
At the end of iteration ΣYi is calculated, if it is close to 1, results are obtained. If not, new value of P is estimated such that ΣYi is close to 1. In excel it can be achieved by using GOAL SEEK function, in which P value is changed to make summation equal to 1.
Note
For some initial values of Pressure, Yi become equal to Xi and summation ΣYi becomes 1, it happens when initial guess for P falls in critical region. For such cases use lower value of pressure such that summation is not equal to 1 and then use Excel GOAL SEEK function to estimate Bubble Point Pressure and vapor mol fractions Yi.
Resources
- Spreadsheet for Bubble P Flash using PR EOS