Estimating Binary Interaction Parameter by Regression
Wilson equation is commonly used to predict non ideality in binary mixture vapor liquid equilibrium. This article shows how to estimate binary interaction parameters used in wilson equation from experimental data by regression in excel spreadsheet.
Example
Determine binary interaction parameters used in wilson equation for a mixture of acetone and chloroform from T x-y experimental data available at 101.33 kPa.
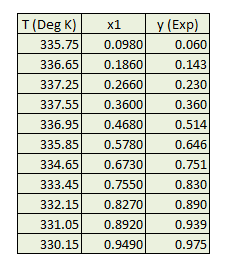
Obtain pure component properties of acetone and chloroform from literature mainly vapor pressure data and liquid molar volume.
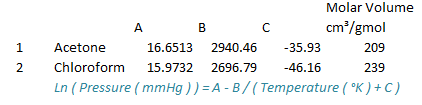
Based on modified Raoult's Law following relation is obtained.
yi.P = xi.γi.Pisatyi = xi.γi.Ki
From above equation experimental γ1 is obtained for acetone.
γ1(Exp) = y1/ ( x1.K1 )K1 = [ e(A - B/ (T + C))] / P
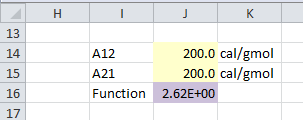
An initial value of binary interaction parameter is assumed.
A12 = 200 cal/gmolA21 = 200 cal/gmol
Liquid phase γ1 is obtained from above interaction parameter and using wilson equation.
Y12 = V2/V1.e-A12/RTY21 = V1/V2.e-A21/RTlnγ1 = -ln(x1 + (1-x1)*Y12) + (1-x1)[ Y12/(x1 + Y12.(1-x1)) - Y21/(1 - x1 + Y21.x1) ]
Square of difference of γ1_experimental and γ1_calculated is obtained for all data points. An objective function is defined as summation of all these differences.
Click on Solver in Data Ribbon (Excel 2010) to open dialog box for Solver parameters and input data as shown below.
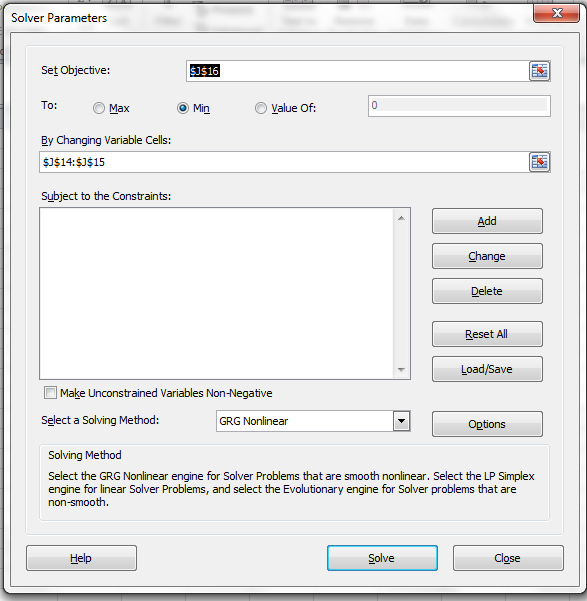
Minimize the objective function by changing values of A12, A21. "Uncheck Make Unconstrained Variables Non-Negative", as these variables can take negative values. Click solve to start regression and new values of A12 and A21 are calculated.
A12 = 157.9 cal/gmolA21 = -570.3 cal/gmol
Above values are then used to calculate y1 values and results are plotted to check the deviation.
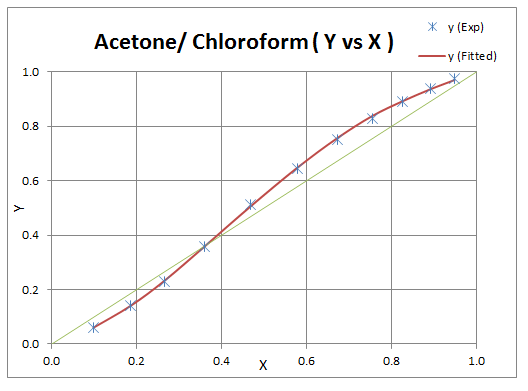
Example
Determine binary interaction parameters used in wilson equation for a mixture of benzene and acetonitrile from P x-y experimental data available at 318.15 °K.
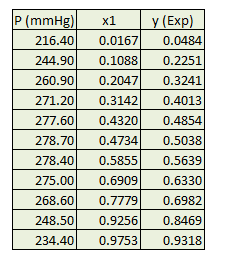
Use above steps and change formula for Y12, Y21 as temperature is fixed. After doing regression binary interaction parameters are obtained and result is plotted as following.
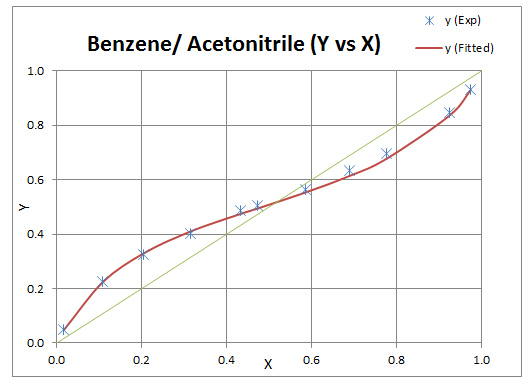
Resources
- Spreadsheet for Binary Interaction Parameter